WHAT IS CARBON COMPOUND?
WHAT IS ORGANIC COMPOUND ?
An organic
compound is any member of a large class of gaseous, liquid, or solid chemical
compounds whose molecules contain one or more atoms of carbon which are
covalently linked to atoms of other elements, most commonly hydrogen, oxygen,
or nitrogen. Mostly known as hydrocarbon.
WHAT IS INORGANIC COMPOUND ?
An
inorganic compound is a chemical compound which is not an organic compound.
That means it is not a carbon-based compound. Example :
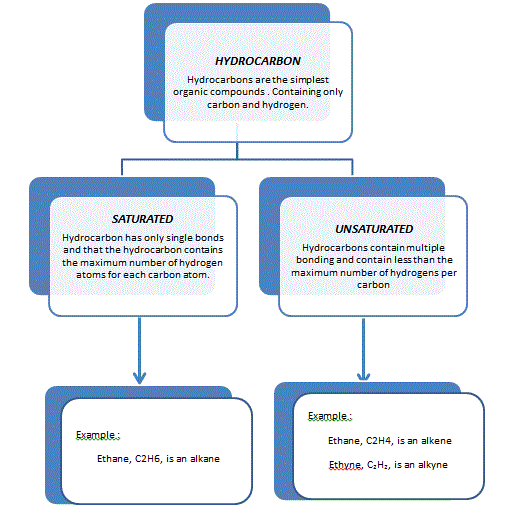
WHAT IS HYDROCARBON?
SOURCES OF HYDROCARBON

The most abundant sources of alkanes are natural gas
and petroleum deposits, formed over a period of millions of years by the decay
of organic matter in the absence of oxygen. Natural gas contains 60–80 percent
methane, 5–9 percent ethane, 3–18 percent propane, and 2–14 percent higher
hydrocarbons. Petroleum is a complex liquid mixture of hundreds of
substances—including 150 or more hydrocarbons, approximately half of which are
saturated.
COMBUSTION
OF HYDROCARBON
Hydrocarbons
can have many chemical bonds between the atoms and, when burned, the bonds are
broken which releases tremendous heat energy. The simplest chemical reaction
from the combustion of hydrocarbons releases water (steam), carbon dioxide and
heat energy. Can occur in excess or limited oxygen.
The
flammability of hydrocarbons makes them very useful as fuels, and they are the
primary energy source for today’s civilization. Worldwide, most electricity is
generated by the burning of these compounds, and they are used to propel
practically every mobile machine: cars, trucks, trains, planes, and ships. They
are also used in the manufacture of many other chemicals and materials. Most
plastics, for example, are hydrocarbon polymers. Other uses include solvents,
lubricants and propellants for aerosol cans.

















0 comments:
Post a Comment