ISOMERISM
Existence of different compounds with the same molecular formula but different structural formular. Depend to branching of carbon and location of functional group.
ISOMERS
Different compounds that have same molecular formula
There are two types of isomers;
2. Positional Isomer -
the basic carbon skeleton remains unchanged, but the functional groups are moved around on that skeleton.
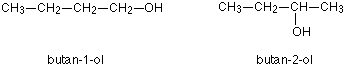
Isomerism in alkanes
Isomerism in alkenes
the basic carbon skeleton remains unchanged, but the functional groups are moved around on that skeleton.
Isomerism in alkanes
| Molecular formula | Number of isomers | Structure name |
| CH4 | (no isomer) | Methane |
| C2H6 | (no isomer) | Ethane |
| C3H8 | (no isomer) | Propane |
| C4H10 | 2 | Butane 2-methylpropane |
| C5H12 | 3 |
Pentane
2-methylbutane
2,2-dimethylpropane
|
Isomerism in alkenes
| Molecular formula | Number of isomers | Structure name |
| C2H4 | (no isomer) | Ethene |
| C3H6 | (no isomer) | Propene |
C4H8
|
3
|
But-1-ene
But-2-ene
2-methylpropene
|
C5H10
|
5
|
Pent-1-ene
Pent-2-ene
2-methylbut-1-ene
3-methylbut-1-ene
2-methylbut-2-ene
|
















0 comments:
Post a Comment